High-Temperature Impact is a critical factor influencing the reliability and lifespan of lithium-ion batteries, a reality underscored by extensive electrical performance testing. From high-temperature storage and cycling to thermal chamber evaluations, these assessments reveal that even moderate heat can trigger detrimental changes—including cell swelling, increased internal resistance, and material softening. Extreme conditions may lead to thermal runaway, a destructive failure mode, but the gradual performance decline under normal high temperatures is equally concerning. To address this challenge, it is essential to unpack the root causes of battery degradation driven by High-Temperature Impact.
How High-Temperature Impact Alters Cathode Materials
Cathode materials exhibit varying resilience to High-Temperature Impact, with their crystal structure stability being a defining factor. The general order of high-temperature performance is: Lithium Iron Phosphate (LFP) > Lithium Cobalt Oxide (LCO) > Medium-Low Nickel Ternary > High-Nickel Ternary ≈ Lithium Manganese Oxide (LMO). Among these, high-nickel ternary materials are particularly vulnerable to High-Temperature Impact during storage, with three key degradation mechanisms at play.
First, byproduct accumulation occurs. High temperatures promote side reactions on the surface of high-nickel ternary materials, leading to the formation of a rock-salt phase. This non-electrochemically active phase increases battery impedance, hindering ion and electron transfer. Second, transition metal dissolution and deposition take place. Heat causes transition metals (e.g., nickel, cobalt) to leach from the cathode, which then deposit on the anode’s graphite surface. This deposition damages the Solid Electrolyte Interphase (SEI) film, accelerating the consumption of active lithium ions critical for energy storage. Third, surface and bulk structure transformations are observed. While X-ray Diffraction (XRD) analysis shows no significant changes in the bulk crystal structure of cathode materials after high-temperature storage, High-Resolution Transmission Electron Microscopy (HRTEM) reveals the growth of a thicker rock-salt phase on the surface and the formation of spinel phases in local grain regions—both reducing reversible capacity.
X-ray Photoelectron Spectroscopy (XPS) further confirms the role of High-Temperature Impact in electrolyte decomposition. Electrolytes containing lithium hexafluorophosphate (LiPF₆) break down at elevated temperatures, producing byproducts such as LixPOyFz, LixPFy, and LiF. These compounds deposit on the cathode surface, replacing portions of the polyvinylidene fluoride (PVDF) binder and further increasing internal resistance.
To mitigate High-Temperature Impact on high-nickel ternary cathodes, surface coating with solid electrolytes is effective. This coating minimizes electrode-electrolyte interface reactions, stabilizes the material surface, and inhibits transition metal dissolution—protecting the anode’s SEI film and preserving active lithium. Bulk doping, commonly with aluminum (500–5000 ppm in industrial applications), stabilizes the layered structure of high-nickel materials, preventing phase transitions to rock-salt or spinel phases. However, this method may slightly reduce the material’s capacity. In contrast, LFP’s inherently stable crystal structure makes it highly resistant to High-Temperature Impact, explaining its superior high-temperature performance.
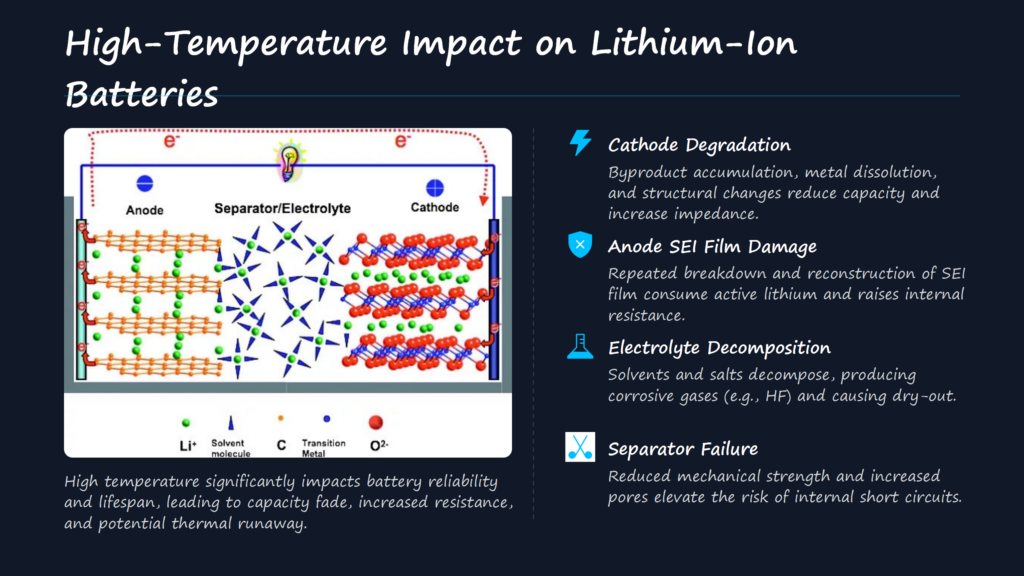
High-Temperature Impact on Anode Materials: The Critical Role of the SEI Film
While High-Temperature Impact has a relatively minor direct effect on anode materials themselves, its primary damage mechanism targets the SEI film—a thin protective layer on the anode surface. Several factors contribute to SEI film degradation under heat: corrosion by dissolved transition metals from the cathode, formation of hydrofluoric acid (HF) via electrolyte decomposition, and poor film formation caused by electrolyte instability.
The repeated breakdown and reconstruction of the SEI film under High-Temperature Impact consume substantial active lithium and increase battery impedance. For example, graphite anodes outperform silicon oxide (SiOₓ) anodes in high-temperature environments. Graphite’s lower edge-plane work function facilitates smoother charge transfer during initial SEI formation, resulting in a more stable film. In contrast, SiOₓ’s higher work function hinders charge transfer, increasing SEI film solubility and leading to higher self-discharge at elevated temperatures. Scanning Electron Microscopy (SEM) images confirm that graphite electrodes retain their surface integrity after high-temperature storage, while SiOₓ electrodes show partial SEI film dissolution.
To improve SiOₓ anodes’ resistance to High-Temperature Impact, researchers focus on adjusting the material’s surface work function or optimizing the Lowest Unoccupied Molecular Orbital (LUMO) energy level of electrolyte additives. These modifications enhance SEI film stability, reducing lithium loss and impedance growth.
Electrolyte Degradation Driven by High-Temperature Impact
Electrolytes play a pivotal role in battery performance, and their stability under High-Temperature Impact is paramount. Three key components—solvents, lithium salts, and additives—are particularly susceptible to heat-induced degradation.
Solvents with low boiling points and viscosity, such as dimethyl carbonate (DMC) and carboxylates, generate high vapor pressure at elevated temperatures, leading to gas formation that disrupts interface stability. Despite their excellent kinetic properties, these solvents require careful consideration in high-temperature applications. Lithium salts, especially the widely used LiPF₆, exhibit poor thermal stability under High-Temperature Impact, decomposing to release toxic HF. This limitation has spurred interest in alternative salts like fluorosulfonimide (FSI). Additionally, impure lithium salts can trigger severe side reactions at high temperatures, further compromising battery performance.
Additives are critical for mitigating High-Temperature Impact on electrolytes. They react preferentially with electrode materials to form protective films, suppressing side reactions. For instance, sulfur-based additives like polysulfide (PS), polyhydrogen sulfide (PST), and 1,3-dimethyl thiosulfate (MMDS) excel at maintaining high-temperature performance. However, some additives, such as 1,1,2,2-tetrafluoroethylene oxide (ODFB), while beneficial for high-temperature cycling, may cause gas generation during storage, leading to performance degradation.
High temperatures also accelerate interface side reactions and electrolyte dry-out in liquid electrolyte systems. Solid electrolytes address these issues effectively, as their rigid structure reduces side reactions and prevents electrolyte leakage—marking a promising solution for enhancing battery resistance to High-Temperature Impact. Overall, optimizing electrolyte performance requires improvements to solvent systems and post-film-formation interface regulation.

How High-Temperature Impact Affects Battery Separators
Separators, which prevent short circuits while enabling ion transport, are also vulnerable to High-Temperature Impact. Elevated temperatures cause polyethylene (PE) separators to turn yellow when in contact with the cathode, increase gas permeability, reduce mechanical strength, and raise impedance. These changes stem from separator oxidation and the deposition of electrolyte oxidation products.
Two effective strategies mitigate High-Temperature Impact on separators. First, coating separators with alumina (Al₂O₃) or boehmite (γ-AlOOH) neutralizes HF generated by LiPF₆ hydrolysis, inhibiting voltage drop. These ceramic coatings also scavenge singlet oxygen released by the cathode, reducing electrolyte oxidation, and block non-polar [C-O-F-P]-based deposits from accumulating on the separator surface. Second, using PE-based films with high surface smoothness alters the deposition rate of electrolyte polymers, minimizing black substance accumulation on the cathode and separator. Separators with high permeability facilitate the migration of electrolyte oligomers to the anode, reducing their accumulation on the separator and slowing oxidative polymerization.
Comprehensive Solutions to Mitigate High-Temperature Impact
Addressing High-Temperature Impact on lithium-ion batteries requires a holistic approach targeting all core materials—cathodes, anodes, electrolytes, and separators. Beyond material optimization, auxiliary components and manufacturing processes also play critical roles. For example, the specific surface area of conductive agents influences heat dissipation, while binders affect the structural integrity of electrode composites under heat. Strict moisture control during production, optimized formation and aging protocols, and improved mixing processes reduce residual impurities and stress, enhancing battery resistance to High-Temperature Impact.
Research into advanced materials and technologies continues to drive progress in this field. For insights into cutting-edge solid electrolyte developments, refer to studies published by the Journal of Power Sources. For guidelines on battery thermal management systems, the International Electrotechnical Commission (IEC) provides industry standards that help mitigate High-Temperature Impact in real-world applications. By integrating material innovations, process improvements, and thermal management strategies, the industry can develop lithium-ion batteries with enhanced high-temperature performance, meeting the demands of applications ranging from electric vehicles to portable electronics.
Understanding the multifaceted effects of High-Temperature Impact is the first step toward developing more durable, reliable lithium-ion batteries. As research advances, addressing this challenge will unlock new possibilities for energy storage, ensuring batteries perform optimally even in harsh thermal environments.
Recommended products
-
Atomfair LFP-S03 LiFePO4 (LFP) Electrode Sheet- Single Side Coating, 15.6 mg/cm², Wet Process, 5 Pack Sheets
$99.00 -
Atomfair NCM811-D01 lithium nickel cobalt manganese oxide (NCM811) Electrode Sheet-Double Side Coating, 24 mg/cm², Wet Process, 5 Pack Sheets
$99.00 -
Atomfair NCM811-D04 lithium nickel cobalt manganese oxide (NCM811) Electrode Sheet-Double Side Coating, 60 mg/cm², Dry Process, 5 Pack Sheets
$139.00 -
Atomfair 1AH LFP||Graphite Dry Pouch Cell Without Electrolyte Filling
$99.95 -
Atomfair 1AH NCM811 || Graphite Dry Pouch Cell Lithium Ion Battery
$99.95



